An overview of cancer research
Cancer research has led to the medical treatments and health programs available today.
These advances have improved outcomes for people with all types of cancer over the past 20 years, with increases in both length of survival and quality of life. The search for better ways to prevent, diagnose and treat cancer is ongoing.
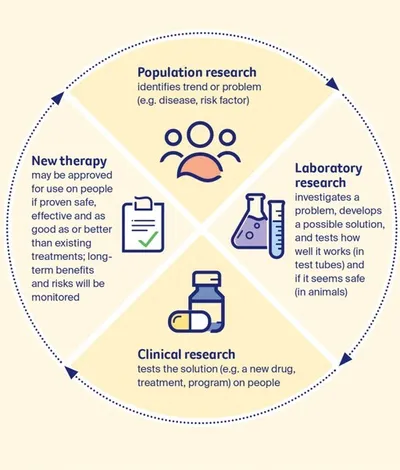
There are three main types of cancer research:
- population research – researchers known as epidemiologists look for patterns and trends to work out how and why cancers occur in groups of people (populations)
- laboratory research – scientists do experiments with the building blocks of disease, such as cells and blood, to try to understand how cancer works, and they also study and develop new drugs and treatments in the laboratory
- clinical research – research is done on people to better diagnose, prevent and treat cancer, often in a hospital or treatment centre.
Population research and laboratory research are often the starting point for clinical research, which may involve clinical trials.
What is a clinical trial? – A clinical trial can help show whether a new approach to prevention, screening, diagnosis or treatment works better than current methods and is safe. People volunteer to help test how well the new way works and if it causes side effects or other problems. If the new way is shown to work better than the existing method, it may become available. Some clinical trials compare existing approaches to see which one is more effective.
Cycle of research
The research process is a continuous cycle. People affected by cancer mainly take part in clinical trials, a type of clinical research. Research can lead to new and improved therapies becoming part of standard care.
Key questions
Answers to some key questions about clinical trials and cancer research are below.
Why get involved in research?
When cancer patients, carers and survivors, as well as people not affected by cancer, take part in research, it helps researchers learn more about cancer and ways to treat and prevent it.
Many people diagnosed with cancer who join a clinical trial or another research study do so because they want to help improve outcomes for others in the future, as well as for themselves.
Adults and children can take part in different ways, including:
- consenting to their medical records and personal information being accessed
- doing surveys and interviews
- being involved in a clinical trial
- agreeing to be examined regularly by health professionals
- allowing samples of cells or tissue taken during tests or treatment to be used for research outside of their own medical care.
Who can participate in research?
All research studies, including clinical trials, have guidelines setting out who can take part. These are known as the eligibility criteria.
Most cancer research involves current patients, but some studies focus on cancer survivors, carers, family members, people at risk of cancer or people who have not been affected by the disease. Anyone under the age of 18 needs permission from a parent or guardian before joining a research study.
To make sure results reflect Australia’s diverse population, it is important that research involves people of all ages, genders and sexualities, as well as people from a wide range of social, economic, racial and cultural backgrounds.
Where does research take place?
Cancer research is carried out in many places, including hospitals, treatment centres, laboratories and universities.
Sometimes you can be involved in cancer research from home. For example, you might have treatment or medicines mailed to you, or you might be asked to fill in a survey or complete a telephone or face-to-face interview.
Tele-trials for cancer
In Australia, some people are now taking part in clinical trials that use telehealth. This means that the research team use telephone or video calls to talk with patients and with local health professionals who are delivering part of the trial.
Clinical trials that use telehealth are sometimes called tele-trials. They were developed to make joining a clinical trial easier for people in rural and remote locations, but they can make it more convenient for anyone to take part.
You can talk to your cancer specialist about whether there is a tele-trial you could join. If you are already involved in a clinical trial, you could ask if parts of it can be delivered by telehealth.
Who works on clinical trials?
A team of people work on clinical trials, and some of their roles overlap. If you decide to join a clinical trial, you may have contact with all or some of the people listed below.
In most cases, your cancer specialist will continue to look after your overall cancer care while you are on the clinical trial.
Investigator
- also known as a researcher – there are usually a number of investigators/researchers involved in a clinical trial
- develops and plans research studies, and obtains, analyses and publishes the results
- may have a background in medicine, nursing, science, psychology, allied health, consumer advocacy or complementary therapies
- the principal investigator is responsible for conducting the trial at their hospital or university, ensuring patients are safe and the trial is properly run; is usually a doctor or senior academic with expertise in the field of research
- a co-investigator works in partnership with the principal investigator in helping run the trial
- the coordinating principal investigator oversees research that is taking place at more than one study site, e.g. at two or more hospitals
Nurse or research assistant
- may be called a clinical trials nurse, a clinical research nurse or a research assistant
- coordinates finding people for the trial (recruitment) by talking to potential participants, making sure they are eligible and explaining the purpose of the trial
- arranges appointments for tests, treatments or to see the specialist
- makes sure all necessary paperwork is completed once you have agreed to join a trial
- provides emotional support
- acts as a link between the patient and the researchers or the health care team
- may also be the main contact person for the trial
- larger clinical trials or hospitals have a dedicated clinical trials nurse, but smaller ones might not
Study coordinator
- may be called a clinical research coordinator or clinical trials coordinator
- may be combined with the nursing role
- may have a science or nursing degree (or similar)
- ensures the trial meets ethical and legal requirements
- applies for grants and manages budgets
- reviews the detailed plan (protocol) for the trial and organises records
Cancer specialist
- may be a medical oncologist, surgeon, radiation oncologist or haematologist
- supervises your treatment, follow-up and overall care
- usually is also the principal investigator or the coordinating principal investigator
Other professionals
- a pharmacist stores and dispenses trial medicines, monitors their effect on patients and provides advice; may conduct laboratory research
- allied health practitioners and complementary therapists may give advice or treatment if the study is investigating non-medical treatments such as counselling, nutrition, massage, physiotherapy and acupuncture
Will I get better care in a clinical trial?
There are many advantages to being involved in a clinical trial or other research study. Depending on the type of research, the benefits may include:
- knowing you have made a valuable contribution to helping others in the future
- joining programs or having medicines or other treatments that are not readily available outside of the study and may be better than the current standard treatment for that disease
- getting access to expensive drugs that your specialists recommend but that are not currently subsidised by the Pharmaceutical Benefits Scheme (PBS)
- seeing your treatment team, including specialists, more often
- taking an active role in your health care
- learning new ways to improve your lifestyle
- improving or maintaining your quality of life
- feeling that you have tried all treatment possibilities.
Your doctor and the clinical trials or research nurse will discuss the possible advantages and disadvantages for you before you join a research study. Taking part in research doesn’t always mean you will be better off than before or compared to other people in a similar situation. Some people may not respond in the way researchers hope and will not benefit from being involved in the research.
In some clinical trials, people are divided into two groups. Only one group receives the experimental treatment, while the other group receives the current standard treatment. You won’t get to choose which treatment you have, but either way you will be monitored more frequently and closely than usual.
Is research safe?
Understandably, people want to know if there are any risks to taking part in research. Researchers must follow strict guidelines to make sure clinical trials and other research studies are as safe as possible for everyone involved. This is called their duty of care.
Before any research involving people can begin, it must be approved by a special group known as a human research ethics committee. As part of this process, researchers identify risks that might occur, such as possible side effects. They must also explain how they will closely monitor these risks and what will be done if problems occur. Before you agree to take part in research, you must be told about the risks, how you will be monitored for problems, and what will be done to help you if problems occur.
To reduce the risks, clinical trials are arranged in a series of steps known as phases.
How long do studies last?
From start to finish, clinical trials and other research studies may take several months or many years, but you may only need to be involved for some of this time. For example, you might just have a single appointment lasting a couple of hours, or you may go to appointments every few weeks, months or years. You may also have to do surveys at regular times. The participant information will set out what you would need to do and how long you would be involved.
Studies have what is known as a recruitment phase. This involves finding people to enrol in the study. It usually occurs over a few months or years until the required number of people have agreed to take part. The study is then closed to new participants.
After the treatment stage is over, there may be a follow-up phase. People may be followed up at set intervals for months or years. This allows researchers to understand the long-term effects of treatments, monitor the general health of the participants, and collect data about long-term survival and quality of life.
Can I still have other treatments?
Ask your doctor whether being involved in the research will affect any other treatments you’re having or planning to have. These may include standard cancer treatments; medicines for managing treatment side effects or symptoms of cancer or other conditions; and complementary therapies such as herbal or nutritional supplements or massage.
Your doctor may suggest stopping or delaying some treatments, or adjusting them in some way (e.g. by changing the dose).
It is important to let the research team know about any other medicines, supplements or complementary therapies you are having, as these may interact with the treatment being tested and cause harmful side effects.
Is it free to join a study?
Joining a clinical trial or other research study is free for Australian citizens and residents, and you should not be asked to pay to join.
The cost of trial-related treatment, tests and check-ups will be paid for by the organisation that is funding or conducting the research, sometimes called the sponsor. You will usually still have to pay for any treatments or tests you would normally pay for as part of your standard care. The participant information will outline any extra costs to you.
Will I be paid?
People participating in cancer research usually don’t get paid. This is because offering people money to join a clinical trial may put too much pressure on them to agree.
In some circumstances, you may be paid back for certain expenses (e.g. for travel, parking, light refreshments). These are known as out-of-pocket costs. The participant information will outline what expenses will be covered.
Can I be involved in more than one research study?
You may be interested in joining multiple clinical trials and other research studies. Check with the research team whether you can be part of more than one study at the same time. If you can, think about whether you’ll be able to commit to all the requirements of the studies. With clinical trials for medicines, you can usually only join one trial at a time.
Clinical trials explained
Cancer clinical trials are research studies that use volunteers to test new ways (interventions) to diagnose, treat and manage cancer.
If a trial proves that a test, treatment or other intervention is better than existing options, it may become the new standard of care for patients in the future.
A medicine or another intervention can be developed in a laboratory and tested on animals, but it is only once it has been tested on the human body that we can know it works in people. It also has to be tested on enough people to show that any benefit is not just a random effect for a single person.
A clinical trial will be designed to answer a particular type of research question.
Treatment trials – These test new treatments, new ways of giving existing treatments, or new combinations of treatments. They look at whether the treatment works and if it causes side effects. The treatments that can be tested include: medicines (chemotherapy, immunotherapy, targeted therapy and other drugs); medical devices; radiation therapy; surgical techniques; nutrition advice; physiotherapy; exercise programs; psychological therapies; and complementary therapies.
Most cancer clinical trials in Australia are treatment trials. The information in this section mostly relates to treatment trials.
Prevention trials – These work out whether medicines and health programs lower the risk of developing diseases such as cancer.
Screening trials – These look at new methods of detecting diseases before symptoms appear.
Diagnostic trials – These identify more accurate or easier tests for diagnosing a particular disease in people who have signs or symptoms.
Quality of life trials – These test ways to improve the comfort and quality of life of people who have cancer. They are done alongside a treatment trial.
Registry trials
For most treatment trials, you will have the treatment as part of the trial. Another way researchers can compare how well different treatments work is through registry trials. A registry trial collects information from people with a particular type of cancer who are having routine treatment. It can’t be used for testing new treatments, but it can answer a range of questions about existing treatments.
Your cancer treatment team may talk to you about joining a registry trial. If you choose to join the registry, you agree to share your health information, such as your medical history, the treatments you are having and the results of tests. This information will then be compared with information from many other patients to work out which treatment approaches are the most effective.
Registry trials are a cost-effective way to run a clinical trial and they allow many more people to participate in cancer research.
The phases of a clinical trial
Researchers spend many years developing new treatments or medicines in the laboratory before involving people. They then plan the clinical trial to progress in a series of steps called phases. Every phase considers whether the risks outweigh the benefits. Information gathered in each phase determines whether the study can move on to the next phase, and whether the drug or treatment is approved for use. There can be up to four phases, but not all clinical trials go through every phase.
How clinical trial phases work
Phase 1 – Safety
How many people take part (participants) = 10-100
What it aims to do (purpose)
- first study in people
- tests safety of new treatment
- finds the safest dose and the best way it can be given, identifies side effects and checks how the treatment works with other medicines or food (interactions)
- often includes people with different types of cancer
How it works
- participants are given a fixed dose and watched closely for side effects
- the dose is often small in the first group of participants, and then gradually increased in the next groups until any side effects outweigh the potential benefits
More information
- may involve overnight stay in hospital for monitoring
- people may benefit but often don’t see big improvements
Phase 2 – Effectiveness
How many people take part (participants) = 100-300
What it aims to do (purpose)
- builds on the results of the phase 1 trial
- continues to test safety of the new treatment
- begins to assess how well the new treatment works on the disease
How it works
- often focuses on one type of cancer
- the participants may be put into separate groups and given different treatments (randomised controlled trial) or they may all have the same experimental treatment (non-randomised trial)
More information
- people often have treatment as an outpatient
Phase 3 – Comparison
How many people take part (participants) =100s-1000s
What it aims to do (purpose)
- tests if the new treatment is better than the best currently available treatment (standard treatment)
- compares side effects, survival and quality of life
- collects information that allows new treatments and existing treatments to be used in new ways or for different diseases
How it works
- uses two or more treatment groups – experimental and control
- usually groups are randomised and sometimes blinded
- a placebo may be used for comparison
- runs over a longer period of time
More information
- some phase 3 trials help the Therapeutic Goods Administration (TGA) work out whether to approve a new drug for use in Australia
Phase 4 – Follow-up
How many people take part (participants) = 1000s
What it aims to do (purpose)
- identifies how well the new treatment works when used more widely in the real world
- monitors the long-term benefits and risks
- looks for other uses of the treatment
- sometimes known as an expanded access study
How it works
- carried out after the treatment has been approved by the TGA
- usually run by pharmaceutical companies that make the product
- some people are able to get early access to new drugs before the cost is covered by the Pharmaceutical Benefit Scheme (PBS)
More information
- not all treatments go through a phase 4 clinical trial
- phase 4 trials are less common than phase 1–3 trials
"When I was diagnosed with cancer, a friend told me about the study. Getting involved was simple. Participating in cancer research is about giving to other people, and I think that’s a very valuable thing.” PHILLIPA
Randomised controlled trials
It is important for researchers to know that the results of a study are accurate and not caused by chance. This means they must follow strict guidelines. Researchers also need to make sure their own – or the participants’ – ideas or beliefs about the research don’t unfairly influence the results. There are various ways to make sure clinical trials are fair and reliable.
Many clinical trials are randomised controlled trials (RCTs). A randomised controlled trial helps prevent bias, so it is the best way to test if a new treatment works. (Bias occurs when the results of a trial are affected by human choice, expectations or other factors not related to the treatment being tested.)
Most phase 3 trials and some phase 2 trials are randomised. This means participants in the study are put into two or more groups (known as treatment arms) at random. The two groups then receive different treatments, and the results of the different groups are compared. Researchers cannot choose who goes into each group.
Test or experimental arm – This group is given the new treatment that is being tested. Sometimes, the experimental treatment is given in addition to the current standard treatment.
Control arm – This group receives the current standard treatment for the disease or an inactive treatment known as a placebo.
When randomly allocated groups are compared with each other, it is possible to work out which treatment is better. This is because researchers can be certain that the results are related to the treatment, and not to any other factors.
How randomisation works
Standard treatment and placebos
In a randomised controlled trial, people in the control arm may receive the standard treatment or a placebo.
Standard treatment – This is the current most effective treatment given to people for their disease or condition. For example, the standard treatment for early breast cancer is surgery, often followed by chemotherapy, radiation therapy and/or hormone therapy.
In some cases, the current standard of care is for the doctors to monitor the cancer closely with regular tests and check-ups, and only offer treatment if the cancer progresses. This approach may be known as active surveillance or watchful waiting. For example, active surveillance is recommended for some early thyroid cancers that aren’t causing any symptoms and are low risk, since the risks of treatment may outweigh the benefits in this situation.
Placebo – This is an inactive or “dummy” treatment. It is made to look, taste or feel like the treatment being tested, but it doesn’t have any active (therapeutic) ingredients (if a medicine) or beneficial effect. Examples of placebos are sugar pills and saline injections.
A placebo is used to show whether any improvements are because of the actual treatment or because of other factors linked with being in the study, such as being more closely monitored or simply expecting the treatment to be helpful. If the people on the experimental treatment improve more than those on the placebo, this provides stronger evidence that it is the experimental treatment that is responsible.
Participants will be told if a study uses a placebo, but they won’t be told which treatment they are having, and the research team usually don’t know either. In cancer treatment trials, placebos may be used:
- together with the standard treatment – for example, one group gets the existing standard therapy plus the experimental treatment, and the other group gets the standard treatment plus a placebo
- on their own, but only when there is no existing standard treatment to compare against an experimental treatment.
Blinded studies
In a blinded study, participants don’t know which arm of a study they’re in. Some randomised controlled trials are called double-blind studies as neither the participant nor the research team know who is receiving the experimental or control treatment. In a double-blind trial, the researchers only discover who is in each arm of the study at the end of the trial when the results are being analysed.
Blinding is used only when participants can’t tell the difference between the two types of treatment. It is not used when the control and experimental treatment are noticeably different – for example, it would be hard to hide surgery and massage from the participant.
The aim of blinding is to reduce bias in the reporting of benefits and side effects. If you don’t know which treatment you’re having, the results are less likely to be influenced by your or your doctor’s views. For example, if you or your doctor knew you were having the experimental treatment, you might report that you’re feeling better than you actually are because you believe you are having a more effective treatment. In an emergency, your doctor can find out what treatment you’re having by contacting those running the study.
Crossover studies
In crossover studies, the people in each trial arm receive that treatment for a time and may then have the opportunity to swap to the other treatment. This can mean all participants have all treatments and helps confirm which is the most effective. If the new treatment doesn’t work as hoped with the first group, the second group won’t cross over. You also won’t cross over if you are doing well on the first treatment.
Non-randomised trials
In a single arm trial, everyone receives the same experimental treatment. This method may be used for phase 1 and phase 2 trials, or where the cancer being treated is rare and it is hard to conduct a randomised trial.
Other types of clinical research
Clinical trials are not the only type of clinical research studies. You may also be invited to get involved in behavioural research or translational research.
Behavioural research
Behavioural researchers try to understand why people behave in the way that they do. They study the characteristics, lifestyles and social situations of different people to see how these factors may affect the risk of developing or surviving cancer. They then try to develop ways to prevent or change behaviours that might increase cancer risk or lower survival outcomes. Behavioural researchers also look at the emotional and social impacts of cancer on the person with cancer, as well as the impact on their family and friends.
If you take part in a behavioural research study, you may be asked to fill in surveys or be interviewed about your lifestyle, including your eating, drinking, smoking and exercise habits.
You may also be able to take part in a program aimed at changing these behaviours. For example, you might be offered free counselling, an exercise class or a session on healthy eating. The aim of the programs may be to reduce cancer risk or to improve how you cope with the impacts of cancer.
Psychosocial research
One area of behavioural research is called psychosocial research. This looks at how cancer affects people emotionally, psychologically and socially. In cancer care, this is sometimes called psycho-oncology. Researchers try to understand how patients and carers cope emotionally at different stages of a disease. They develop and test ways to improve people’s ability to deal with the impacts of cancer.
Translational research
Translational research forms a link between laboratory and clinical research, or between clinical research and standard treatments. It aims to get new treatments or medical approaches into practice quickly. It is sometimes called “bench to bedside” research because laboratory research results are directly used to create new therapies and tests. The findings of behavioural and psychosocial research are also translated into information resources for people affected by cancer.
There can also be “bedside to bench” research – for example, hospitals, treatment centres and health professionals may collect information about how well a treatment works to help laboratory researchers work out what to study next.
Joining a trial or study
This section explains how to find out about a clinical trial or other research study and what happens once you decide to participate.
Finding a trial or study
There are many ways to find out about clinical trials and other research studies. Most cancer specialists know about current studies and may recommend you join a suitable study, or you can ask them if there are any trials you can consider. If your hospital has a clinical trial or research nurse, you can also ask them about current studies. You don’t have to join a study at your current treatment centre – your doctor can refer you to a suitable trial at another centre.
Hospital and treatment centre waiting rooms often have information about current studies, or you might hear about a study through patient support groups, in the general media or through social media (such as Facebook). Before providing any personal information, especially via social media, check the study with your doctors.
You can also search clinical trials websites. If there isn’t a suitable study available right now, you can register through websites such as CART-WHEEL, and they will tell you about any studies that come up in the future.
If you find a trial or study you’re interested in joining, discuss it with your cancer specialist. You can ask them if you meet the eligibility criteria and, if so, whether they could coordinate your involvement or put you in touch with the research team.
Deciding to take part
You may have many questions when deciding whether to join a clinical trial. As well as talking to your doctor and clinical trials nurse, it is a good idea to talk to people close to you, such as your family, carers or close friends. Ultimately, though, it’s your decision to participate in research or not to participate.
You shouldn’t feel pressured to take part in research, and you should not feel rushed into making any decisions that may affect your health or treatment. Ask your doctor or nurse how much time you have to think about whether or not to join a study. You should usually be given at least a few days to consider the participant information. If you would like to take more time to think about it, ask if it is safe to wait a bit longer before starting treatment. If you decide not to join a study, you will still receive the standard treatment for the cancer, and it won’t affect your relationship with your treatment team.
Weighing up the benefits and risks
- Consider what is most important to you. Some people prefer to have standard treatment with predictable side effects, whereas others want to try something new even if the benefits are not yet proven or it may have unknown side effects.
- Think about how the study might affect your health, wellbeing and lifestyle. What is the chance of any serious side effects? Will any parts of the study be too difficult (e.g. having to have extra tests or trips to the hospital)?
- Weigh up these drawbacks and risks against the possible benefits, such as a possibly longer survival time or not having to experience certain side effects.
- Remember that everyone’s situation is different – what is the right decision for someone else may not be right for you.
"A clinical trials nurse accompanied me at every stage of the process. She explained what was happening and answered any questions I had.” MARG
Getting a second opinion
Some people like to get a second opinion about whether they should join a research study, particularly if it is a clinical trial. A second opinion can:
- confirm or clarify your doctor’s recommendations
- help you to consider all the advantages and disadvantages of being on the trial
- reassure you that you have thought about the different issues that might affect you.
If you would like to get a second opinion, ask your general practitioner (GP) for a referral to another cancer specialist, but keep in mind that you may have to wait several weeks for an appointment and you may have to pay extra.
You can also ask your cancer specialist if it is possible to talk to another specialist within the same hospital or treatment centre. Some people also like to discuss the possibility of a trial with their GP.
Eligibility criteria
Before any clinical trial or other research study begins, researchers develop the protocol for that study. This detailed plan describes the study’s design, the reasons for doing the study, and who can join (known as the eligibility criteria).
The eligibility criteria are the rules for who can take part in the research study. They outline the features that must be shared by all participants to ensure that the people taking part are similar in certain ways. This is so the results of the study will be clearly related to the treatment, not to other individual factors.
Depending on the research, the eligibility criteria may include:
- age or sex
- cancer type
- stage of the cancer
- symptoms or side effects experienced
- length of time since diagnosis or treatment
- previous treatments for the cancer
- other medical conditions.
Eligibility criteria can be broken down into:
- inclusion criteria – the features a person needs to have to join a research study
- exclusion criteria – the features that stop someone from taking part.
The eligibility criteria also help keep people safe by considering things that might make the trial too risky for them. For example, you may be excluded from a trial if you are pregnant, have high blood pressure, or have some other condition that increases the risks of the treatment.
Although there are thousands of different clinical trials occurring around the world at any one time, eligibility requirements can be very specific, and there may not be a trial suitable for your particular situation. For example, if you have metastatic cancer, the disease may have to be active or progressing for you to be eligible to join a particular trial.
Informed consent
Before becoming part of a clinical trial or other research study, all participants have to give “informed consent”. This means you will be asked to confirm you have read and understood the purpose, risks and possible outcomes of the research before deciding to join. Informed consent involves:
Written information – After talking to you about the study, members of the research team will give you a written document known as the participant information. This written information explains the purpose of the study, what is expected of you if you join, what the possible risks are, how your information will be used, and how the results will be presented. You should read the participant information carefully and note down any questions you have for your doctors and the clinical trials or research team.
Informed consent discussion – After you have read the participant information, you can discuss the study in more detail with the clinical trials or research team. You should ask them to explain any parts of the written information that seemed unclear to you.
Agreement in writing – Once all your questions have been answered, you can then decide whether or not to join the study. If you do choose to take part, you will be asked to sign the informed consent form. This confirms that you understand the purpose of the study, what is involved, the potential risks and benefits, and you agree to participate.
For people under 18, a parent or guardian has to give legal consent as well. Signing the form is not a contract and you can change your mind at any time. You will receive a copy of the consent form signed by you and the researcher.
The process of informed consent continues throughout the study. If the study changes or new information becomes available while you are involved, you will be given updated participant information. You will need to sign an updated version of the consent form if you are willing to continue.
Sometimes you may need to consent to each aspect of a study. For example, you might agree to take part in a trial of a new surgical procedure, and then need to consent for your tissue to be collected and banked during that surgery. You might also need to consent again if you are given an extra survey or interview.
For some low-risk studies, written informed consent may not be needed. For example, if you are doing a survey, just completing the survey may be taken as consent, or you may be able to say you consent over the phone.
Contact person
All clinical trials and other research studies have a contact person. You can talk with this person before you decide to take part and at any stage during the study if you have questions or concerns. The contact person is often a clinical trials nurse or study coordinator.
You will also be given details of who to contact if you have a complaint about the study (e.g. about how the study was run or how you were treated). This person is independent of the research team and is usually a senior member of the human research ethics committee. Complaints about research are rare, but it is your right to have your concerns heard if you have a problem.
Participant information
Researchers must provide written information about the research study to anyone thinking about joining. This is called participant (or patient) information. It answers a range of questions about the study, including:
- the purpose of the study
- if it is a clinical trial, and what phase it is in
- who can participate in the study
- who is running the study (institution and researchers)
- who has approved the research
- who is funding the study
- how the study will be run and what you need to do
- how the study will be monitored
- whether you will need to have tests, scans or other procedures
- how long you need to be involved
- where you need to go for appointments, treatments or meetings
- whether the researchers need to see your medical records
- whether you will be paid back (reimbursed) for any expenses related to the study (e.g. transport or parking costs)
- information about possible side effects or other risks
- information about possible benefits
- any restrictions on things you can do while you are on the study (e.g. treatments you can’t have)
- who to contact for more information or if you have any problems or complaints during the study.
- information about your rights, such as keeping your records private and your ability to withdraw from a study
Privacy
Medical records are private and confidential, including those about your involvement in a clinical trial or any other research study.
Health professionals directly involved in your care or study can access your personal and medical information, but only if it’s necessary for their work. They can’t give any information about you to others unless it is relevant to your health care or the study.
Clinical trials and other research studies collect personal data about you. The participant information may mention who will and won’t have access to your personal data. For example, it might state that your regular treatment team won’t see your survey responses, but the researchers will. You might be asked to consent to the research team seeing your existing medical records or particular test results.
Information collected during the study is often de-identified. In most cases, this means that your name will be removed and replaced with a unique participant number so your identity cannot be revealed unless necessary, e.g. for safety reasons. Being de-identified means when the results are published in medical journal articles or discussed at medical conferences, you will not be named.
If you have questions or concerns about your privacy in a clinical trial or other research study, talk to the clinical trials or research nurse. For general information about privacy issues in health care, talk to the social worker at your hospital.
At the end of a clinical trial, all personal information is stored in a secure place for at least 15 years before it can be destroyed. This is a legal requirement.
"My doctor suggested I take part in a study, and I thought it sounded beneficial. I found the thorough disclosure of both the trial and the possible side effects reassuring.” PIERS
Your role in the trial or study
What you need to do when you agree to join a clinical trial or other research study depends on what kind of research it is. Some studies need preparation and ongoing follow-up, others involve a single visit or just a survey.
Your participation is usually organised by one person (often a clinical trials or research nurse or research assistant), but you may come into contact with different members of the research team.
Finding out about the trial
- Your cancer specialist may suggest a trial to you, or you may find a trial through your own research.
- Discuss the trial with a member of the clinical trials team and your cancer specialist. You may also choose to see another specialist for a second opinion.
Checking you are eligible
- Answer some questions about your medical history and have a physical examination and any required tests, such as a CT scan and blood test, to check that the trial is suitable for you. This is known as screening.
- Once the clinical trials team has these results, they will let you know if you have been accepted into the trial.
Deciding to join
- Read the participant information. You may want to discuss the information with family, friends or your GP.
- Ask the trials team any questions you still have about the study, including questions about safety, side effects, financial issues, number of visits and timing.
- If your questions have been answered and you agree to take part in the trial, sign the informed consent form.
What to expect
- Expect that you may have less flexibility on the trial than you would with standard cancer care – for example, you may need to have blood tests or treatment on particular days. This may mean you need to adjust your work schedule or childcare arrangements more than you would if you were not involved in the research.
- Be prepared for more tests and visits to your doctor than you would normally have. This is to monitor your health and to see if and how the treatment is working.
During the trial
- Follow the instructions you are given about the trial – that means going to all appointments, having the required tests, taking medicines at precise times, and completing logs, surveys or interviews. This will help make the trial results as reliable as possible.
- Tell the research team about any side effects you are experiencing. The research team will ask about how you are feeling emotionally and physically.
After the trial
- Researchers may stay in contact with you and ask follow-up questions for some time after the trial. This will give them long-term information on how you responded to the trial.
- You may return to having the standard care and/or check-ups and tests that are appropriate for you, depending on the stage of the cancer and what your cancer specialist recommends. In certain circumstances, you may be able to keep having the trial treatment.
Communicating with the treatment team
- Keep contact details handy in case you have questions before, during or after the trial or study.
- If your clinical trials team has given you a participant card, keep this with you and show it to any other health professionals you see.
- If you join a clinical trial at a different hospital to the hospital where you started cancer treatment, you may have two treatment teams. In this case, make sure your medical information and any relevant test results are available to both treatment teams, and ask who your main contact person is.
- If you are in a trial and develop new or worsening symptoms, let your clinical trial and cancer care teams know immediately. If the symptoms are serious, go directly to your hospital’s emergency department and/or contact the oncology registrar at the hospital. It is important to tell the hospital you’re in a clinical trial and show them the participant card if you have one. If you go to hospital, let your clinical trials team know.
Continuing the treatment
Many people wonder if they can keep having the experimental treatment after a trial is over. This depends on several factors, such as the trial phase and results; how effective the treatment was for you; what the recommended course of treatment is; and whether the trial sponsor is prepared to continue providing the treatment.
Some people join clinical trials to get treatments that are not available anywhere else. It can be frustrating to have to stop a treatment after the study ends. When a trial shows that the experimental treatment works and has no major side effects, the treatment can sometimes be continued after the trial. Ask your doctor or clinical trials nurse.
Withdrawing from a clinical trial
Participating in research is voluntary and you can stop (withdraw) at any time. You may want to leave because you no longer have the time or energy for it; don’t feel it is helping; have side effects or your health is worsening; move away from the treatment centre; or change your mind.
If you do decide to withdraw from a clinical trial, you will receive the standard treatment that is currently the best option for you. You may be asked why you are withdrawing from the trial – you do not have to give a reason, but it can help the research team understand more about the needs of participants if you do. You may also be asked if the team can still collect some information about your health. This can help them learn more, but it is your choice.
Finding trial results
It can take a while to get trial results. Usually results are available 2–5 years after the study finishes, but sometimes it can take 10 years or more. The results of most clinical trials will be published in medical journals and presented at medical conferences and scientific meetings.
If you’d like to know the results of the study you participated in, start by asking your doctor or clinical trials coordinator. The participant information you read at the beginning of the trial and the informed consent document you signed often say how and when the results will be available. The results are usually reported for everyone in the trial together, so you may not be able to see your individual results.
You may want to talk to your doctor about what the overall results mean. Sometimes the study results are shared with participants in an easy-to-understand document (lay summary).
Cancer research in Australia
Australia is an international leader in cancer research and has contributed to many of the major advances in cancer prevention, treatment and support. The involvement of people affected by cancer has been the key to much of this progress.
Before any clinical research can begin, it has to show it is scientifically worthwhile and fair to the people participating. The organisation, institution or company responsible for the overall conduct of a clinical trial is known as the trial sponsor. Their responsibilities include developing, financing and managing the trial, and ensuring the trial meets all legal, ethical and insurance requirements.
Who funds cancer research?
Between 2012 and 2020, more than 4800 cancer research projects and programs were funded in Australia, with a total value of $2.12 billion. The Australian Government was the largest funder of these projects and programs, providing 58% of the direct funding. Cancer Council is one of the largest non-government funders of cancer research in Australia.
Funding for cancer research comes from sources including:
National Health and Medical Research Council (NHMRC) – This is the Australian Government’s main funding body for medical research. The NHMRC awards grants to researchers based on their ability to investigate important questions about human health. The NHMRC also helps run the Medical Research Future Fund.
Medical Research Future Fund (MRFF) – The Australian Government set up this ongoing research fund in 2015, and it had grown to $22 billion by December 2023. The fund earns interest that can then be used to pay for important health and medical research projects.
Cancer charities – State and territory Cancer Councils and other charities receive donations from the public, as well as grants from government bodies and private companies. This funds their own cancer research and allows them to financially support cancer research carried out by other institutions.
Government bodies – Researchers can apply for grants from other national, state and territory government agencies. The grants fund the research and allow them to employ cancer trials staff.
Universities, medical research institutions and clinics – These sometimes use their own funds and staff to support research.
Private companies (industry funded) – Companies producing medicines and medical equipment run trials to check their products are safe and effective before applying for licences to sell them. Private companies may also fund research in partnership with a university or other research institution, or for goodwill (philanthropic) reasons.
Are there rules for research with people?
All research involving people must follow a set of international standards. These standards cover how the research is designed, conducted, recorded and reported. They ensure that people taking part in the research are safe, their privacy is protected, information is collected to the highest standard, and the results are reliable.
In addition, clinical trials and other clinical research must follow a set of standards called Good Clinical Practice (GCP). GCP is the same anywhere in the world where clinical research is conducted.
For links to more information about Australian guidelines for research involving people, visit Australian Clinical Trials.
How research is approved
Before a clinical trial or other research study can begin in Australia, it has to be approved by a number of committees:
Research or scientific review committee – This committee decides whether the study has social and scientific value and checks that it has been designed to produce valid scientific results.
Human research ethics committee (HREC) – This committee confirms that the interests of participants are protected and that researchers will run the study in a fair, honest and neutral (impartial) way. It ensures researchers won’t force people to participate, and that the risks of the research generally don’t outweigh the benefits.
The members of the HREC are always independent of the researchers and come from a variety of professions and the general community.
Research governance review – This is a review that checks all the locations (sites) where the research will take place. It is done by a research governance officer, who makes sure there are enough resources to carry out the proposed research and that the staff members involved are qualified. The governance officer approves the research to begin at each site.
The study may also be monitored by outside agencies such as pharmaceutical companies, independent advisory committees, research institutions and auditors. These bodies ensure that the research is carried out properly.
Conflict of interest
A conflict of interest may arise in any research project. This means the interests of an organisation or researcher could influence the results of the research. Researchers are required to declare any possible or actual conflicts of interest. All research projects should include details of how a conflict of interest will be managed.
Changes to research
Sometimes changes need to be made to the research before or after the research is approved. The HREC may ask the research team to make changes to the proposed research before it can go ahead.
After the research has been approved, if the research team want to make any changes during the trial or study, it must go through ethics approval again before the changes can take place. If the changes are made, participants already taking part may need to sign another consent form to show that they have been told about the changes and still agree to be involved.
The Therapeutic Goods Administration (TGA) is an Australian Government department that regulates all medicines sold in Australia. The TGA must be notified of any new drug that is going to be used in a clinical trial. If the trial shows that the drug is safe and effective, the TGA may then approve it for use outside of a clinical trial.
Other ways you can get involved in research
Sometimes people want to contribute to research without joining a clinical trial or other research study. They may have been affected by cancer in the past – either directly or indirectly – and want their experience to help shape future cancer research. Researchers refer to these people as “consumers” to distinguish them from patients or others recruited to a study.
The role of consumers in research has increased over the years. Consumers are now involved in identifying priorities for research and helping to decide which projects should be funded. They may also be asked to review a draft participant information sheet or provide feedback on what a proposed study is asking of participants.
Cancer Council offers an online training course known as the Consumers in Research Training. This teaches people how to work with researchers.
Cancer Australia developed the National Framework for Consumer Involvement in Cancer Control. This initiative includes a web-based toolkit for cancer researchers and other professionals who involve consumers in their work.
The Australian Clinical Trials Alliance offers a consumer toolkit as well as information about clinical trials in community languages.
Consumer advocacy organisations are available at a national level and in some states. They focus on improving cancer treatment through active consumer participation. To learn more, visit them online at:
If you would like to get involved in research as a consumer, contact one of these organisations or call Cancer Council 13 11 20.
Useful websites
You can find many useful resources online, but not all websites are reliable. These websites are good sources of support and information.
To find current Australian trials
To find trials for specific cancers and treatments (Multi-site Collaborative Cancer Clinical Trials Groups)
Australasian Gastro-Intestinal Trials Group
Australasian Leukaemia & Lymphoma Group
Australian and New Zealand Urogenital and Prostate Cancer Trials Group
Australia New Zealand Gynaecological Oncology Group
Cooperative Trials Group for Neuro-Oncology
Melanoma and Skin Cancer Trials
Psycho-oncology Co-operative Research Group